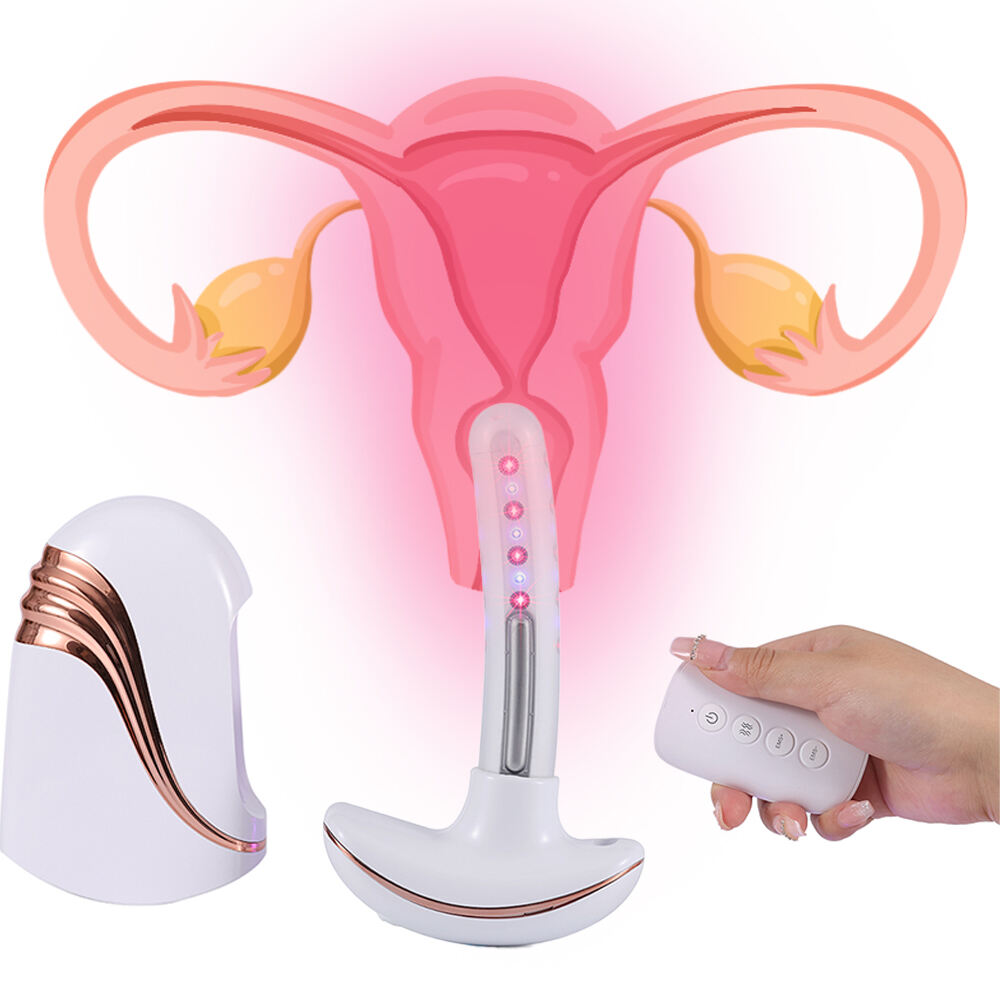
Os produtos ATANG foram aprovados pela FDA (número de registro 3015515517), o que valida suas alegações sobre segurança e eficácia, seguindo as diretrizes de padrões mínimos para um aperto vaginal seguro. Eles também passaram por testes clínicos e análises regulatórias, incluindo testes de segurança a laser SGS e marcação CE. Os produtos ATANG receberam aprovação da FDA, o que garante que sejam fabricados utilizando padrões industriais precisos de qualidade, assegurando, assim, uma experiência sem preocupações para os usuários. Em comparação com concorrentes altamente regulamentados, oferecemos produtos que proporcionam resultados mensuráveis: por exemplo, 91% dos participantes relataram melhora na saúde vaginal após o período de 12 semanas com o dispositivo. Para resultados válidos, utilize produtos aceitos pela FDA.