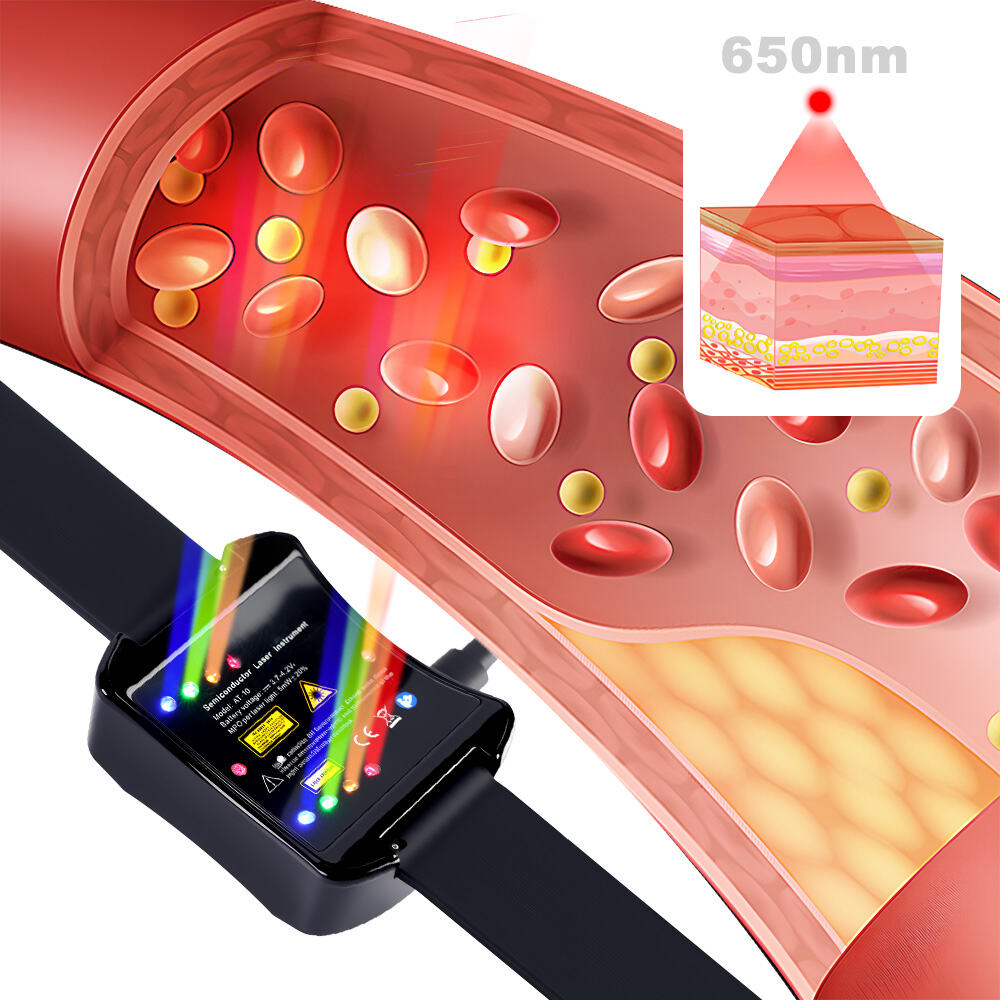
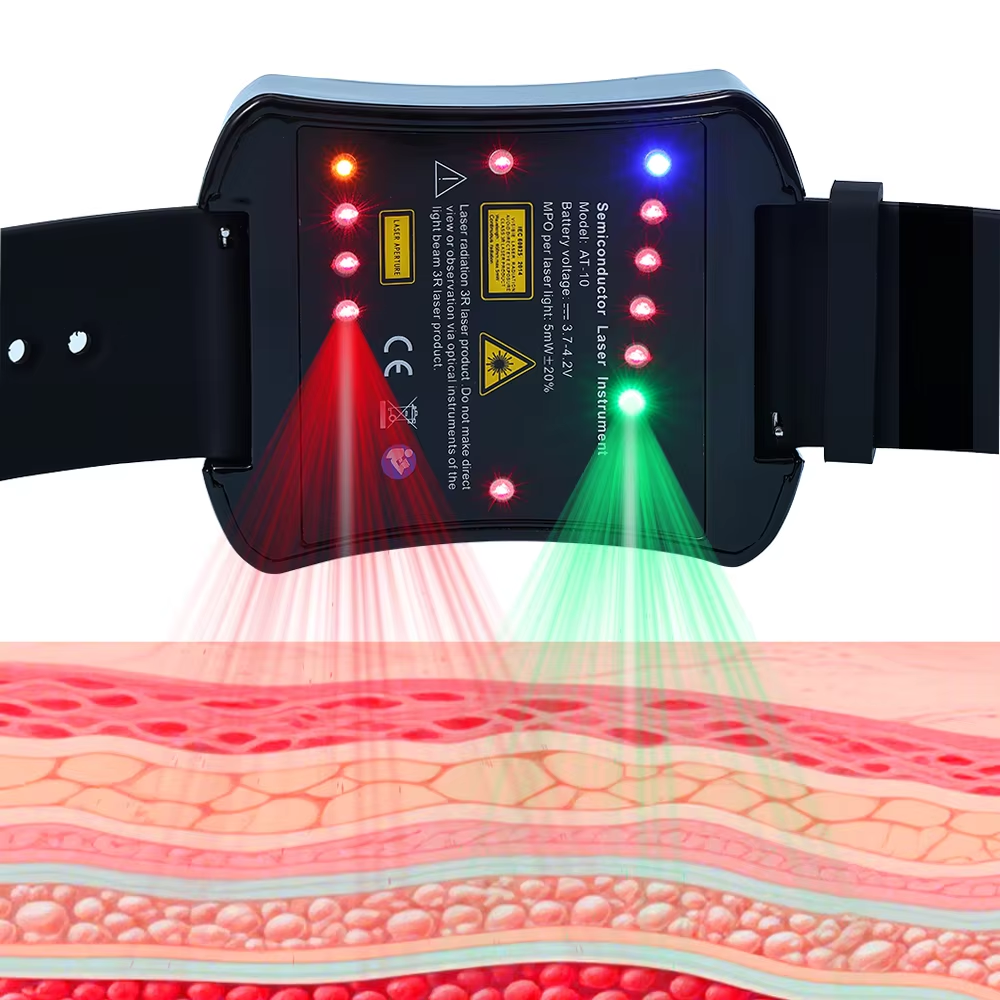
Safety is ATANG’s number one concern with their device for stroke symptoms which has FDA Registration No: 3015515517 and CE markings. Their device features non-invasive low-level 650nm laser therapy which is completely side effect free and increases the functionality of blood vessels and neurological processes. Unlike surgical procedures and medication, this therapy offers gentle relief from fatigue, limb numbness, cognitive impairment, and other stroke symptoms. It is safe and easy to operate due to its ergonomic controls and patented technology. Clinically-tested low-level laser radiation also assures its safety. Beyond post stroke recovery, ATANG’s device can be also used proactively, and its durability ensures peace of mind for its users.