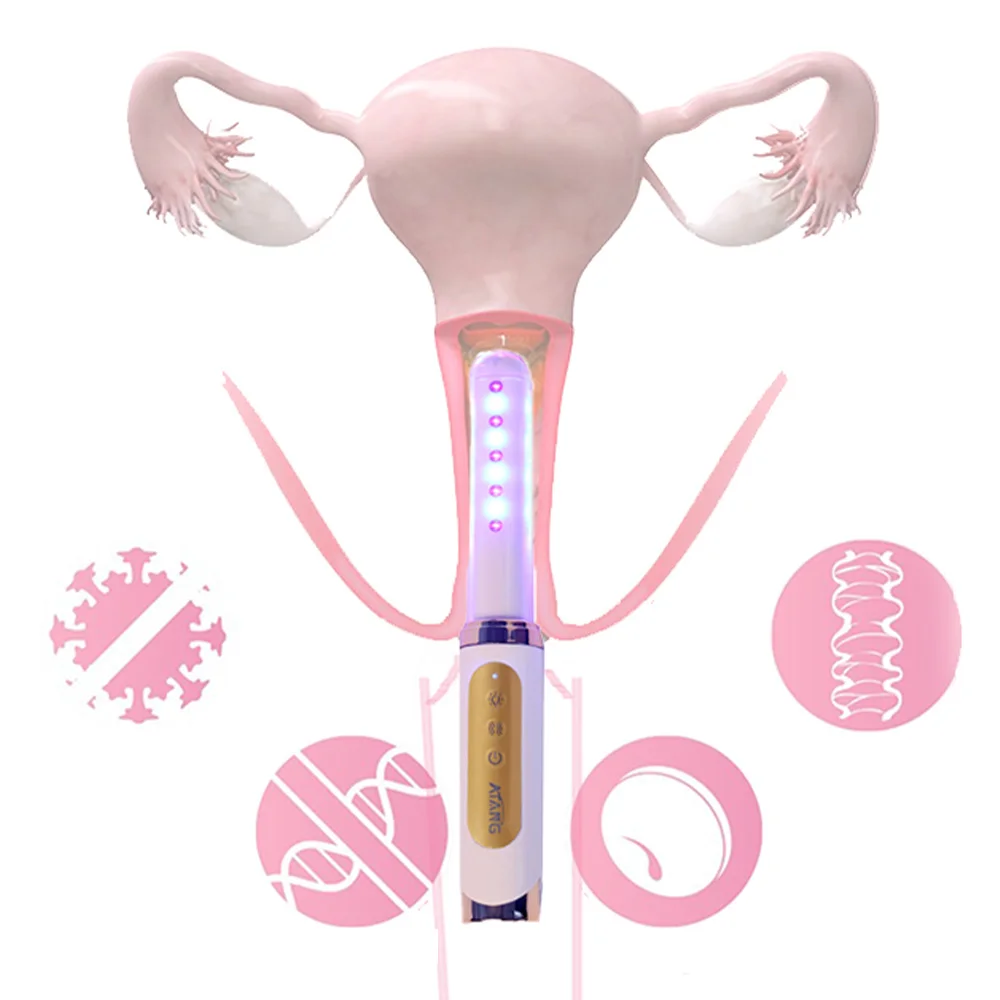
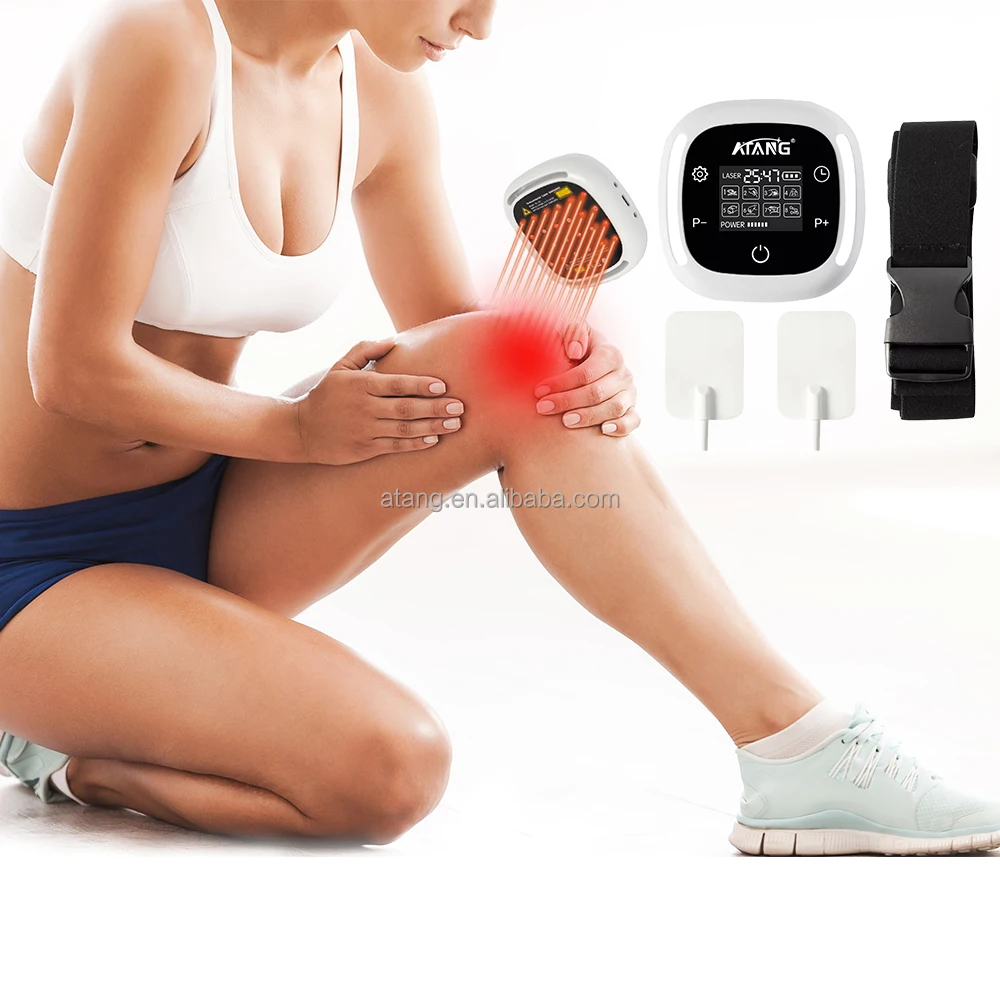
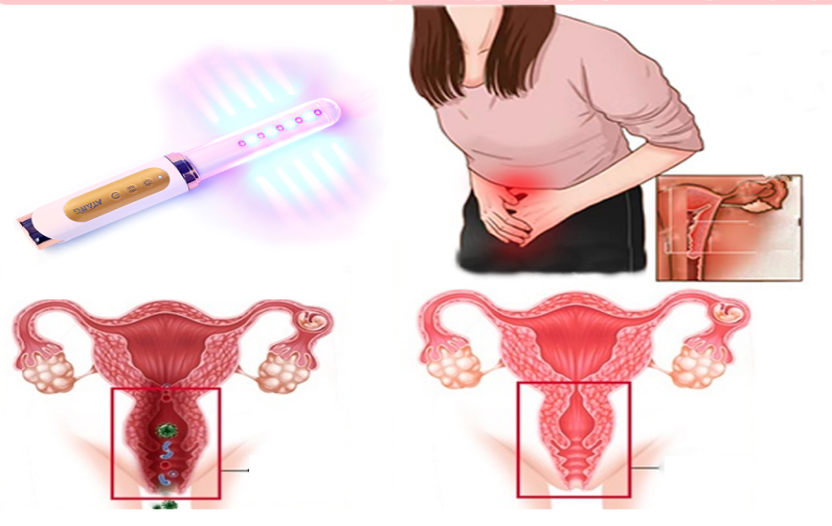
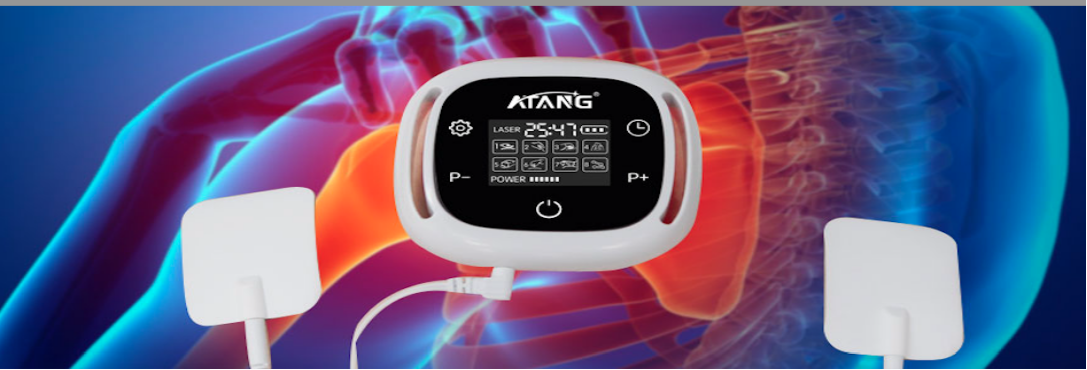
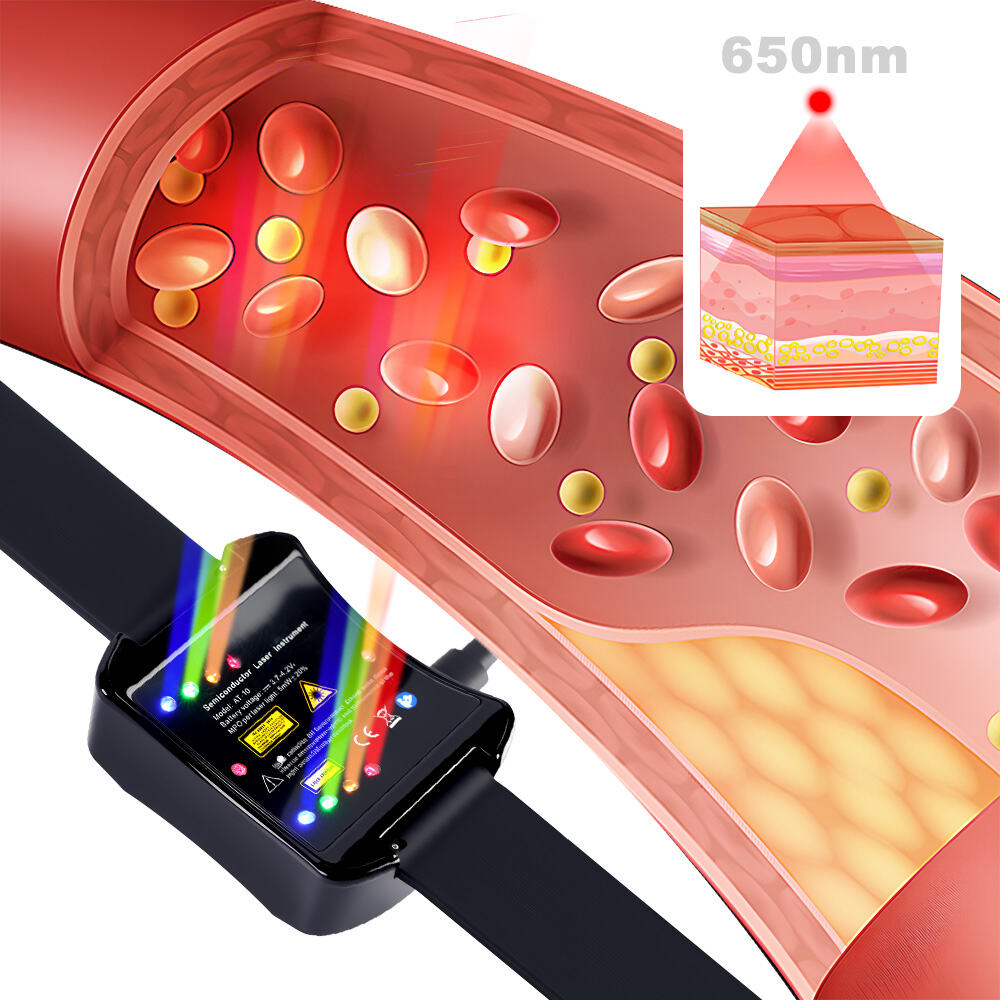
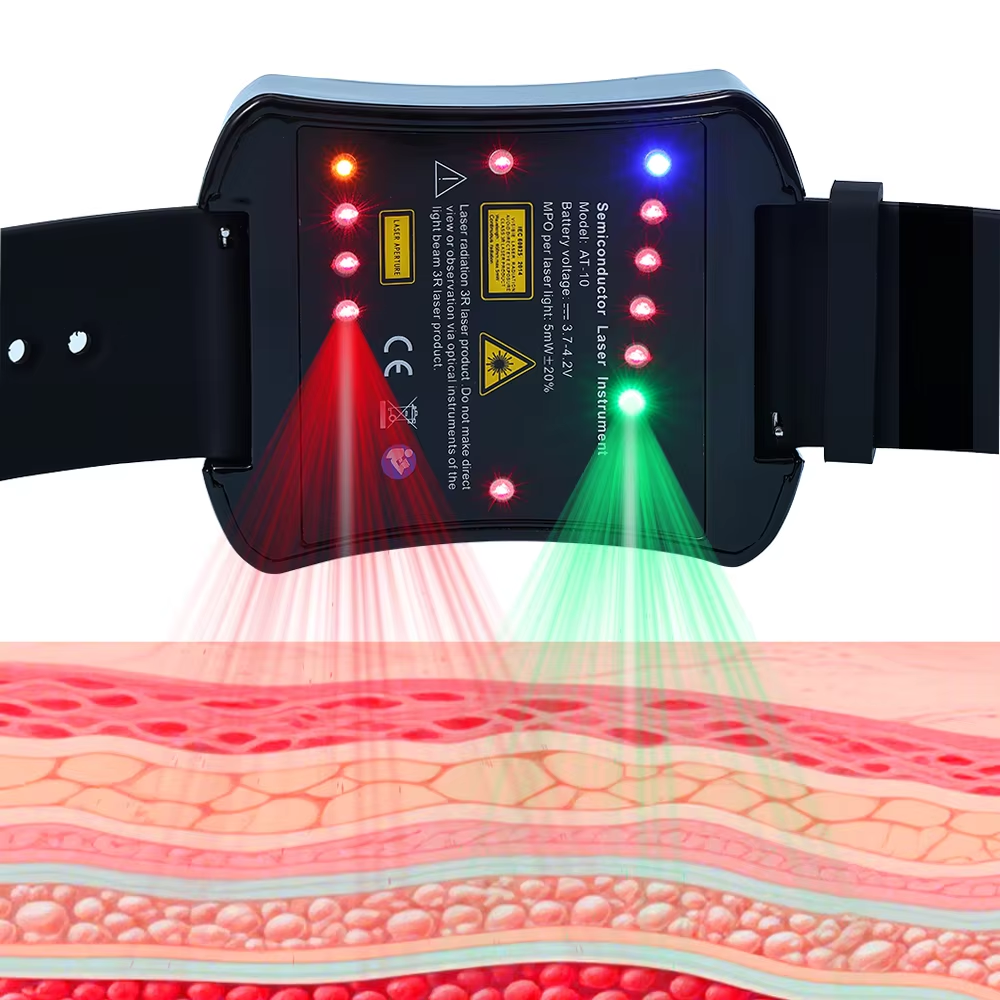
How Laser Therapy Devices Are Changing Diabetes Management
The Increase in case of Diabetes worldwide is making it harder to manage. Diabetes management techniques like Insulin treatment and lifestyle modifications have been the traditional approach to dealing with Diabetes. The introduction of technology ha...
View More